Design and synthesis of a novel 5-(aminomethylene)thiazolidine-2,4-dione derivatives as potent hepatitis-B virus polymerase inhibitors
Abstract
A series of novel thiazolidinedione analogues (TZD) were designed and synthesized potent inhibitors of HBV capsid assembly. The synthesis of thiazolidine-2,4-dione derivatives (4a-4o), starting from the condensation of 5-(ethoxymethylene)thiazolidine-2,4-dione (1) with various secondary amines (3) derived from biologically active compounds. The newly synthesized TZD analogues 4a-4o were characterized by 1H NMR, 13C NMR, and MS and evaluated for their anti-HBV activity. Most of the compounds inhibited the expression of viral antigens at low concentration. Six compounds, 4g, 4h, 4l, 4m, 4n, and 4o, demonstrated potent inhibition of HBV DNA replication at the submicromolar range. Of these five initial hits, compound 4o was the most active when compared with lamivudine.
Introduction
Worldwide more than two billion people were seriously affected by hepatitis B virus (HBV), which can cause Liver cirrhosis and hepatocellular carcinoma in humans. It was estimated that 400 million people were chronically infected with HBV worldwide up to recent times, and HBV was responsible for 750,000 deaths each year (Custer et al., 2004). HBV infections in developing countries increase abnormally. The current therapies including vaccines, immunomodulators, interferon-alpha, polyethylene glycol interferon-alpha and nucleoside drugs for treating HBV are still unsatisfactory, due to high recurrence, drug resistance and inevitable side effects including influenza-like illness, myalgia, headache, reduction of neutrophilic granulocyte and blood platelet, etc (Sato et al., 2010; Locarnini et al., 2006; Wong et al, 1993; Fattovich et al, 1988). Therefore, it is interesting to explore novel classes of drugs with different antiviral targets and mechanisms for anti-HBV purposes.
On the other hand, the thiazolidinedione analogues were investigated for the treatment of a variety of diseases, e.g., as anticancer (Havrylyuk et al., 2009), anti-HIV (Rawal et al., 2005), anti-ischemic (Adachi et al., 1999), anticonvulsant (Ergenc et al., 1994), antimicrobial (Piscopo et al., 1989), antihistaminic (Previtera et al., 1994; Diurno et al., 1999), and antidiabetic agents (Carroll et al., 2011; Bruno et al.,2002), 15-hydroxy prostaglandin dehydrogenase inhibitors (Wu et al., 2011; Zidar et al., 2010),inhibitors of MurD ligase (Ha et al., 2012),aldose reductase inhibitors (Ma et al., 2012), for their anti-obesity effects (Bhattarai et al., 2010).
The thiazolidinediones affect genes and the specific gene expression profile induced by each of these molecules resulting in different activities for the thiazolidinedione derivatives. This observation has encouraged many medicinal and synthetic chemists to modify thiazolidinedione chemistry and utilize this core to synthesize numerous novel compounds with various pharmacological and therapeutic activities. The list of effects induced by the thiazolidinedione analogues is far from complete and needs specific attention. However, considering the broad therapeutic potential, this five-membered heterocyclic ring (thiazolidine-2,4-dione 4) will play an important role in future drug design and development.
The successful results by different researchers using thiazolidine core (Stachulski et al., 2011) encouraged us to identify new, potentially active anti-HBV agents. In this paper, we synthesized and evaluated the anti-HBV activity of different thiazolidone derivatives.
Materials and Methods
Chemistry: Chemicals and solvents were purchased either from Fluka or Merck, and all reagents were of analytical grade. Thin-layer chromatography (TLC) was performed on Merck silica gel 60 F254 plates and visualized under UV light. 1H NMR spectra were recorded with a Varian Mercury Plus 400 MHz instrument. 13C NMR spectra were recorded on a Varian Gemini 100 MHz instrument. Signals due to the solvent (13C NMR) or residual protonated solvent (1H NMR) served as the internal standard. All the chemical shifts are reported in d (ppm) using TMS as an internal standard. Multiplicity is indicated by one or more of the following abbreviations: s (singlet), d (doublet), t (triplet), q (quartet), m (multiplet), br (broad); the coupling constants (J) correspond to the order of the multiplicity assignment. Mass spectra were recorded with a PE Sciex model API 3000 instrument. All the reactions were carried out under a nitrogen atmosphere.
Synthesis of 5-(ethoxymethylene)thiazolidine-2,4-dione (1): Pale yellow solid; yield: 98%; m.p.: 83-91°C; IR (KBr): Ï…max 3383, 3137, 3043, 2989, 2432,1721, 1677, 1473, 1392, 1229, 1105, 1010, 867, 740, 691 cm-1; 1H NMR (400 MHz, DMSO-d6): d 8.38 (br.s, 1H), 7.65 (s, 1H), 4.20 (q, J = 5.4 Hz, 2H), 1.40 (t, J = 5.4 Hz, 3H) ppm; ESI-MS: m/z 173.0 (M+1); ESI-HRMS: m/z calcd for C6H7NO3S ([M+H]+) 174.0212, found 174.0219.
General experimental procedure for the synthesis of TZD derivatives (4a-4o): To a suspension of 1 (100 mg, 0.58 mmol) in acetonitrile was added secondary amines (0.58 mmol) in one lot. The reaction mixture was stirred at room temperature for 15-20 min, and the completion of the reaction was monitored by TLC. The precipitated solids were filtered at the pump and dried to obtain the pure compounds in quantitative yields.
(Z)-5-((Piperidin-1-yl)methylene)thiazolidine-2,4-dione (4a): White solid; yield: 98%; m.p.: 96-98°C; 1H NMR (400 MHz, DMSO-d6): d 11.47 (br.s, 1H), 7.55 (s, 1H), 3.46-3.38 (m, 4H),1.58-1.52 (m, 6H) ppm;13C NMR (100 MHz, DMSO-d6): d 168.0, 167.5, 142.9, 85.1, 51.3(2C), 25.8 (2C), 23.2 ppm; ESI-HRMS: m/z calcd for C9H12N2O2S([M+H]+) 213.0696, found 199.0692.
1-((Z)-(2,4-Dioxothiazolidin-5-ylidene)methyl)piperidine-4-carbonitrile (4b): White solid; yield: 98%; m.p.: 125-126°C; 1H NMR (400 MHz,DMSO-d6): d 11.60 (br.s, 1H), 7.60 (s, 1H), 3.60-3.58 (m, 1H), 3.55-3.50 (m, 4H), 2.00-1.88(m, 4H) ppm; 13C NMR (100 MHz, DMSO-d6): d 167.9, 167.6, 142.9, 121.5, 85.4, 40.3, 28.0(2C), 24.7 (2C) ppm; ESI-HRMS: m/z calcd for C10H11N3O2S([M+H]+) 238.0648, found 238.0645.
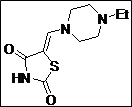
(Z)-5-((4-Ethylpiperazin-1-yl)methylene)thiazolidine-2,4-dione (4c): White solid; yield: 98%; m.p.: 88-89°C;1H NMR (400 MHz, DMSO-d6): d 11.50 (br.s, 1H), 7.56 (s, 1H), 3.45 (t, J = 5.6 Hz, 4H), 2.40 (t, J = 5.4 Hz, 4H), 2.32 (q, J = 4.8 Hz, 2H), 1.00 (t, J = 4.8 Hz, 3H) ppm; 13C NMR (100 MHz, DMSO-d6): d 167.9,167.6, 142.7, 86.0, 52.0 (2C), 51.2 (2C), 49.7, 11.74 ppm; ESI-MS: m/z 242.1 (M+1); ESI-HRMS: m/z calcd for C10H15N3O2S ([M+H]+) 242.0962, found 242.0958.
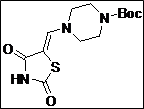
tert-Butyl 4-((Z)-(2,4-dioxothiazolidin-5-ylidene)methyl)piperazine-1-carboxylate (4d): White solid; yield: 98%; m.p.: 72-78°C; 1H NMR (400 MHz, DMSO-d6): d 11.56 (br.s, 1H), 7.60 (s, 1H),3.46-3.38 (m, 8H), 1.40 (s, 9H) ppm;13C NMR (400 MHz, DMSO-d6): d 167.9, 167.2,153.5, 142.8, 86.7, 79.3 (2C), 49.3 (2C), 27.11 (4C) ppm; ESI-HRMS: m/z calcd for C13H19N3O4S ([M+H]+) 314.1170, found 314.1169.
(Z)-5-((((3,4-Dimethoxypyridin-2-yl)methyl)(methyl)amino)methylene)thiazolidine-2,4-dione (4e): White solid; yield: 98%; m.p.: 61-62°C; 1H NMR (400 MHz, DMSO-d6): d 11.40 (br.s, 1H), 8.20 (s, 1H), 7.80 (s,1H), 4.60 (s, 2H), 3.98 (s, 3H), 3.80 (s, 3H), 3.05 (s, 3H) ppm;13C NMR (100 MHz, DMSO-d6): d 168.71, 167.54, 148.65, 145.7 (2C), 145.5, 142.8, 128.7, 108.4, 86.9, 60.4, 56.0 ppm; ESI-HRMS: m/z calcd for C13H15N3O4S ([M+H]+) 310.0852, found 310.0856.
(Z)-5-((Methyl(naphthalen-1-ylmethyl)amino)methylene)thiazolidine-2,4-dione (4f): Off-white solid; yield: 90%; m.p: 81-82°C; 1H NMR (400 MHz,CDCl3): d 11.58 (s, 1H), 8.02-7.84 (m, 4H), 7.62-7.58 (m, 3H), 7.40 (s, 1H), 5.10 (s, 2H),3.15 (s, 3H) ppm; 13C NMR (100 MHz, CDCl3): d 168.5, 167.3, 144.6, 133.4, 131.4 (2C),130.5, 128.7, 128.3 (2C), 126.6 (2C), 126.1 (2C), 125.5, 122.9, 87.0 ppm; ESI-HRMS: m/z calcd for C16H14N2O2S ([M+H]+) 299.0858, found 299.0849.
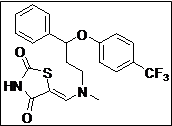
(Z)-5-((Methyl(3-phenyl-3-(4-(trifluoromethyl)phenoxy)propyl)amino)methylene) thiazolidine -2,4-dione (4g): White solid; yield: 88%; m.p.: 101-102°C; 1H NMR (400 MHz, CDCl3): d 11.50 (s, 1H), 7.60-7.58 (m, 3H), 7.40-7.22 (m, 5H),7.10 (s, 2H), 5.50 (s, 1H), 3.56-3.44 (m, 2H), 3.10 (s, 3H), 2.30-2.10 (m, 2H) ppm;13C NMR(100 MHz, CDCl3): d 168.5, 167.2, 160.1, 144.4, 140.2, 128.6 (3C), 127.8, 126.7 (2C), 125.9(3C), 116.0 (3C), 76.7, 54.8, 36.2 ppm; ESI-HRMS: m/z calcd for C21H19N2O3F3S ([M+H]+)437.1160, found 437.1141.
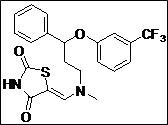
(Z)-5-((Methyl(3-phenyl-3-(3-(trifluoromethyl)phenoxy)propyl)amino)methylene) thiazolidine-2,4-dione (4h): Pale yellow solid; yield: 90%; m.p.: 131-132°C; 1H NMR (400 MHz, CDCl3): d 11.60 (s, 1H), 7.58-7.20 (m, 10H), 5.58 (s, 1H), 3.58-3.28 (m, 2H), 3.00 (s, 3H), 2.30-2.04 (m, 2H) ppm;13C NMR (100 MHz, CDCl3): d 170.0, 157.5, 143.2, 140.3, 130.5, 130.2, 129.9 (2C), 128.6 (2C), 127.8 (2C), 126.0 (2C),119.6, 117.2, 112.4, 109.5, 76.9 ppm; ESI-HRMS: m/z calcd for C21H19N2O3F3S ([M+H]+)437.1162, found 437.1141.
(Z)-5-((Methyl(3-phenyl-3-(o-tolyloxy)propyl)amino)methylene)thiazolidine-2,4-dione(4i): White solid; yield: 96%; m.p.: 129-130°C; 1H NMR (400 MHz, CDCl3): d 11.60 (br.s, 1H), 7.58(s, 1H), 7.40-7.22 (m, 5H), 7.18-7.15 (m, 1H), 7.04-6.90 (m, 1H), 6.78-6.66 (m, 2H), 5.40(br.m, 1H), 4.22 (t, J = 6.6 Hz, 2H), 3.06 (s, 3H), 2.36 (s, 3H), 1.24 (t, J = 6.6 Hz, 2H) ppm; 13C NMR (100 MHz, CDCl3): d 168.6, 167.2, 155.1, 144.4, 141.2, 129.6 (2C), 129.4 (2C),128.5 (3C), 127.6, 126.0 (2C), 115.7 (2C), 76.4, 36.6 (2C), 20.0 ppm; ESI-HRMS: m/z calcd for C21H22N2O3S ([M+H]+) 383.1403, found 383.1424.
(Z)-5-((Methyl(3-phenylpropyl)amino)methylene)thiazolidine-2,4-dione (4j): Pale yellow solid; yield: 90%; m.p.: 117-118°C; 1H NMR (400 MHz, CDCl3): d 11.56 (br.s, 1H), 7.60 (s, 1H), 7.38-7.18 (m,5H), 3.40 (t, J = 5.2 Hz, 2H), 3.10 (s, 3H), 2.62-2.50 (t, J = 7.6 Hz, 2H), 1.90-1.80 (m, 2H)ppm;13C NMR (100 MHz, CDCl3): d 168.5, 167.2, 144.4, 141.0, 128.3 (3C), 128.2 (3C),125.8, 31.8, 29.5 ppm; ESI-HRMS: m/z calcd for C14H16N2O2S ([M+H]+) 277.1014, found 277.1005.
(Z)-5-((4-((2-Chlorophenyl)(phenyl)methyl)piperazin-1-yl)methylene)thiazolidine-2,4-dione (4k): White solid; yield: 89%; m.p.: 92-94°C; 1H NMR (400 MHz, CDCl3): d 7.80 (d, J= 8 Hz, 1H), 7.52 (s, 1H), 7.40-7.20 (m, 8H), 4.80 (s, 1H), 3.50 (br.m, 4H), 2.46-2.20 (m,4H) ppm;13C NMR (100 MHz, CDCl3): d 168.7, 168.6, 142.1, 140.1, 139.2, 132.7, 129.6,128.7, 128.6, 128.5, 128.1, 127.7, 127.4, 87.5, 69.5, 51.2, 49.6, 45.6, 40.1, 38.8, 11.40 ppm;ESI-HRMS: m/z calcd for C21H20N3O2SCl ([M+H]+) 414.1057, found 414.1038.
(Z)-5-((4-((4-Chlorophenyl)(phenyl)methyl)piperazin-1-yl)methylene)thiazolidine-2,4-dione (4l): Off-white solid; yield: 96%; m.p.: 108-109°C; 1H NMR (400 MHz, CDCl3): d 11.60 (s, 1H), 7.58 (s, 1H), 7.56-7.20 (m, 5H), 4.40 (s, 1H), 3.42 (m, 4H), 2.38 (m, 4H) ppm;13C NMR (100 MHz, CDCl3): d 168.1, 167.6, 142.6, 141.6, 141.2, 131.5 (2C), 129.4 (2C),128.7 (2C), 128.6 (2C), 127.6, 127.2, 86.2, 73.2, 51.1 ppm; ESI-HRMS: m/z calcd for C21H20N3O2SCl ([M+H]+) 414.1008, found 414.1038.
(Z)-5-((4-(2,3-Dichlorophenyl)piperazin-1-yl)methylene)thiazolidine-2,4-dione (4m): White solid; yield: 96%; m.p.: 88-89°C; 1H NMR (400 MHz, CDCl3): d 11.60 (s, 1H), 7.62 (s, 1H),7.38 (m, 2H), 7.20 (dd, J = 6.6 Hz, 1H), 3.62-3.60 (m, 4H), 3.16-3.02 (m, 4H) ppm;13CNMR (100 MHz, CDCl3): d 168.0, 150.3, 142.9, 132.6, 128.5 (2C), 126.1, 124.9, 120.0, 86.6,50.7 (4C) ppm; ESI-HRMS: m/z calcd for C14H13N3O2SCl2 ([M+H]+) 358.0186, found358.0178.
(Z)-5-((4-(Benzo[d]isothiazol-3-yl)piperazin-1-yl)methylene)thiazolidine-2,4-dione (4n): Yellow solid; yield: 94%; m.p.: 118-119°C; 1H NMR (400 MHz, CDCl3): d 11.30 (br.s,1H), 8.10 (d, J = 8.4, 1H), 8. (d, J = 8.4, 1H), 7.70 (s, 1H), 7.58 (t, J = 7.2 Hz, 1H), 7.42 (t, J= 7.2 Hz, 1H), 3.76 (m, 4H), 3.58 (m, 4H) ppm;13C NMR (100 MHz, CDCl3): d 168.1,167.8, 162.7, 152.0, 142.9, 128.0, 127.0, 124.4 (2C), 124.1, 121.0, 86.8, 49.3 (4C) ppm; ESI-HRMS: m/z calcd for C15H14N4O2S2 ([M+H]+) 347.0645, found 347.0631.
(Z)-5-((4-(5-Fluorobenzo[d]isoxazol-3-yl)piperidin-1-yl)methylene)thiazolidine-2,4-dione (4o): White solid; yield: 92%; m.p.: 115-117°C; 1H NMR(400 MHz, CDCl3): d 11.58 (s, 1H), 8.15-8.07 (dd, J = 8.8, 1H), 7.76 (dd, J = 8.8 Hz, 1H),7.60 (s, 1H), 7.30 (t, J = 6.8 Hz, 1H), 3.94-3.90 (m, 2H), 3.58-3.20 (m, 3H), 2.20-2.15 (m,2H), 1.90-1.80 (m, 2H) ppm;13C NMR (100 MHz, CDCl3): d 168.0, 164.9, 163.1, 162.4, 160.4, 143.0, 123.8, 117.0, 112.7, 97.4, 86.0, 32.4 (2C), 29.9 (3C) ppm; ESI-HRMS: m/z calcd for C16H14N3O3FS ([M+H]+) 348.0811, found 348.0813.
Biological evaluation
Cell culture: HepG2 2.2.15 cells, derived from HepG2 human hepatocellular carcinoma cells, were stably transfected with a head-to-tail HBV DNA dimer and were maintained in MEM with heat-inactivated 10% fetal bovine serum (FBS) and 1%antibiotics. In parallel experiments, human Huh7 hepatoma cells were maintained in Dulbecco's modified Eagle medium (DMEM) supplemented with heat-inactivated 10% FBS and 1% antibiotics. HepG2 2.2.15 and Huh7 cells were both grown at 37°C in a humidified atmosphere of 5% CO2 and 95%air.
Cell viability assay: The cytotoxic effects of IN-4 (5) were determined using a Cell Titer 96 cell proliferation assay kit (MTS) (Promega, USA). In order to pinpoint the toxicity limits in HepG2 2.2.15 and Huh7 cells, they were plated into 96-well plates at a density of 4 x 104 cells/mL for 24 hours. Cells were then treated with serial dilutions of IN-4 (5), ranging 2.5-160 mg/mL, and the mixture was incubated for 3 days. Cell toxicity was measured according to the manufacturer's protocol. All measurements were performed in four replicates, and results are presented as relative percentages over that of the control group.
Determination of HBV expression levels: After treating HepG2 2.2.15 cells or HBV-transfected Huh7 cells, levels of the HBsAg and HBeAg proteins were measured in culture media using an EIA kit (Johnson and Johnson, USA) according to the manufacturer's instructions.
SEC general procedure: Capsid assembly was initiated by mixing Cp149 with test compounds in 2 buffer incubition 24 hours, respectively. Assembly reactions were examined on a Superose column (Biosep-SEC-S3000) mounted on HPLC system equipped with an auto injection module. The column was equilibrated with 100 mM HEPES pH 7.5, 300 mM NaCl at 0.6 mL/min.
Result and Discussion
The synthesis of a novel series of substituted 5-(aminomethylene)thiazolidine-2,4-diones (compounds 4a–4o) is shown in Scheme 1. In the first step, thiazolidine-2,4-dione 1 was reacted with triethyl orthoformate in the presence of Ac2O at reflux temperature afforded 5-(ethoxymethylene)thiazolidine-2,4-dione 2 as a pale yellow crystalline solid (Lo et al., 1954). The condensation of 1 with various secondary amines in ethanol at reflux temperature resulted 5-(aminomethylene)thiazolidine-2,4-diones in good yields. The secondary amines were obtained from different commercial sources. Thus, the structures of the synthesized target compounds are listed in Table I.
Scheme 1: Synthesis of 5-(aminomethylene)thiazolidine-2,4-diones (a) Ac2O, HC(OEt)3, reflux (b) acetonitrile, 45°C, 30 min
Table I: Synthesis of a novel series of substituted 5-(aminomethylene)thiazolidine-2,4-dionesa
| Entry | 2o Amine (3) | Producta | Yieldb (%) |
|---|---|---|---|
| 1 | 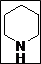 |
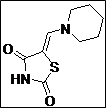 |
98 |
| 2 | 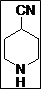 |
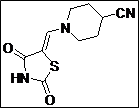 |
97 |
| 3 | 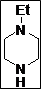 |
 |
91 |
| 4 | 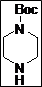 |
 |
98 |
| 5 | 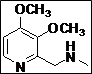 |
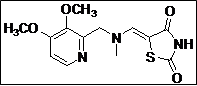 |
96 |
| 6 | 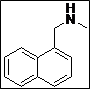 |
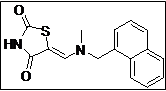 |
91 |
| 7 | 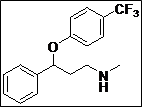 |
 |
87 |
| 8 | 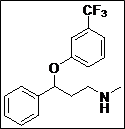 |
 |
92 |
| 9 | 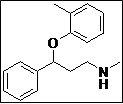 |
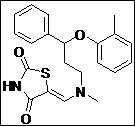 |
95 |
| 10 | 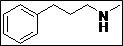 |
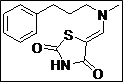 |
91 |
| 11 | 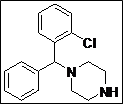 |
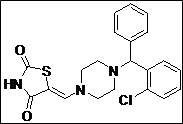 |
88 |
| 12 | 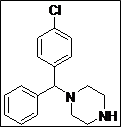 |
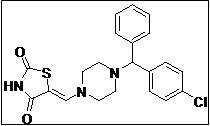 |
97 |
| 13 | 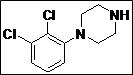 |
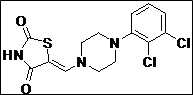 |
95 |
| 14 | 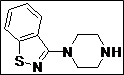 |
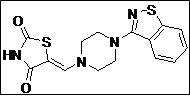 |
93 |
| 15 | 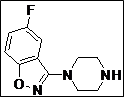 |
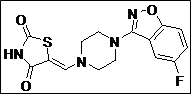 |
90 |
| aCompound 2 (1 mmol.), compound 3 (1 mmol.), acetonitrile (10 vol.), RT, 15-20 min; bIsolated yields | |||
Inhibition activities for HBV replication of the compounds 4a-4o were determined in the HepG2.2.15 cells, which constitutively produces HBV genomes, and secretes virus-like particles (Korba et al., 1992). Lamivudine (3TC) was used as positive control. To ascertain the cytotoxic effects of the tested compounds, the cell viability was determined after the cells exposed to the compounds for 48 hours (Table II).
Table II: Anti-HBV activity and cytotoxicity of target compounds in vitro
| Compound | Cytotoxic activity | Anti HBV activity | ||
|---|---|---|---|---|
| TC50 (uM) | TC0 | TC50 (uM) | SI | |
| 4a | 28.3 | 4.3 | 35.7 | 5.7 |
| 4b | 32.7 | 4.9 | 37.6 | 3.2 |
| 4c | 43.3 | 13.0 | - | - |
| 4d | 77.2 | 10.3 | - | - |
| 4e | 25.7 | 4.2 | 30.2 | 2.5 |
| 4f | 46.8 | 12.8 | 9.6 | - |
| 4g | 29.6 | 3.7 | 7.5 | 13.1 |
| 4h | 96.4 | 14.7 | 6.7 | 6.2 |
| 4i | 41.3 | 6.6 | - | - |
| 4j | 31.2 | 4.6 | 14.3 | 12.3 |
| 4k | 55.2 | 12.8 | 18.3 | 6.3 |
| 4l | 54.0 | 14.0 | 6.7 | 8.1 |
| 4m | 29.7 | 6.3 | 6.3 | 11.8 |
| 4n | 25.8 | 5.9 | 4.3 | 12.5 |
| 4o | 17.4 | 4.1 | 3.2 | 15.3 |
| 3TC | 5.7 | 0.8 | 13.9±1.4 | |
Hep G2.2.15 cell contained multiple copies of the HBV genome, which were stably integrated into the host cell genome and was widely used as a useful in vitro model for evaluation of novel anti-HBV drugs. So, in the experiment, the Hep G2.2.15 cell line as in vitro cellular model was chosen.
All the target compounds were tested in vitro in HepG2.2.15 cells for anti-HBV activity and cytotoxicity. The properties of synthesized compounds are summarized in Table II, in which they are compared to the drug lamivudine. Compounds 4g, 4h, 4l, 4m, 4n, and 4o displayed good to better anti HBV activity.
A comparison of the IC50 values of the derivatives 4a-4o clearly indicated that the size and polarity of substituent on the substitution had noticeable effect on its antiviral activities (Table II). 4l (IC50 = 6.66 µM), bearing a (Z)-5-((4-((4-chlorophenyl)(phenyl) methyl)piperazine group, was more potent inhibitor of HBV replication than 3TC (13.87 µM). When the attachments on TZD was replaced by rigidified piperazinyl derivatives to give compounds 4k, 4m, 4n, 4o (the IC50 values 18.3, 6.3, 4.3 and 3.2 µM respectively), their anti-HBV activities were reserved or even improved. Replacing the piperizine attachment atom of N-methyl side chain containing nucleus in the compounds 4e to 4j there is no improvement in anti-HBV activity. There is no effect on the anti HBV activity when the TZD compound was substituted with piperidine or piperazine derivatives 4a to 4d. In the five piperazinyl derivates, 4k (IC50 = 18.3 µM), 4l (IC50 = 6.7 µM), 4m (IC50 = 6.3 µM), 4n (IC50 =4.3 µM) and 4o (IC50 = 3.2 µM) also showed high inhibition of HBV replication, however, When there is no attachment on piperazine, such as 4c and 4d, both of them gave none antiviral activity. It illuminated that the anti-HBV activity largely depends on the size, length and character of TZD substituent.
Among all the compounds 4m, 4n, and 4o which are showing better anti-HBV activities, compounds containing the electron-withdrawing groups such as fluorine or chlorine are more effective than heterocyclic systems.
To gain better understand into the mechanisms of our compounds, 4o was investigated to examine the effect on preformed HBV capsid and on HBV capsid assembly by size exclusion chromatography (Stray et al., 2005). Recovered protein was assigned to the void (aberrant capsid induced by 4o, 8.5 min), capsid (9.5 min) and dimer (12.5 min) based on the HPLC chromatogram.
By SEC, we had observed the effect of compounds 4o on Cp149 (Figure 1). There was no change of the capsid morphology detected at low concentration of 4o (Cp149:4o = 4:1 or 2:1). At higher concentration (Cp149:4o = 1:2 or 1:4), the increasing continuous spectrum of void and the decreasing dimmers were observed. The results suggested that 4o can break the equilibrium and change the product of HBV core protein self-assembly. By structural biology, we established a screening system for anti-HBV compounds that target on nucleocapsid. SEC would be a much better method to discover the strong antiviral compounds because of its objectivity, convenience and precision.
Figure 1: SEC showed the effect of Mo13 on capsid assembly
Conclusion
The newly synthesized TZD analogues 4a-4o were evaluated for their anti-HBV activity, which provided 5 active derivatives inhibiting HBsAg secretion, 6 active derivatives suppressing HBeAg secretion and 5 active derivatives inhibiting HBV DNA replication. Interestingly, compound 4o could inhibit not only HBsAg and HBeAg secretions but also HBV DNA replication with SI values of 41, 123.4 and 286.
References
Adachi Y, Suzuki Y, Homma N, Fukazawa M, Tamura K, Nishie I, Kuromaru O. The anti-ischemic effects of CP-060S during pacing-induced ischemia in anesthetized dogs. Eur J Pharmacol. 1999; 367: 267-73.
Bhattarai BR, Kafle B, Hwang JS, Ham SW, Lee KH, Park H, Han IO, Cho H. Novel thiazolidinedione derivatives with antiobesity effects: Dual action as PTP1B inhibitors and PPAR-γ activators. Bioorg Med Chem Lett. 2010; 22: 6758-73.
Bruno G, Costantino L,Curinga C, Maccari R, Monforte F, Nicolò F, Ottanà R, Vigorita MG. Synthesis and aldose reductase inhibitory activity of 5-arylidene-2,4-thiazolidinediones. Bioorg Med Chem. 2002; 10: 1077-84.
Carroll RT, Dluzen DE, Stinnett H, Awale PS, Funk MO, Geldenhuys WJ. Structure-activity relationship and docking studies of thiazolidinedione-type compounds with monoamine oxidase B. Bioorg Med Chem Lett. 2011; 21: 4798-803.
Custer B, Sullivan SD, Hazlet TK, Iloeje U, Veenstra, DL, Kowdley KV. Global epidemiology of hepatitis B virus. J Clin Gastroenterol. 2004; 38: S158-68.
Diurno MV, Mazzoni O, Correale G, Monterrey IG, Calignano A, La Rana G, Bolognese A. Synthesis and structure-activity relationships of 2-(substituted phenyl)-3-[3-(N,N-dimethylamino)propyl]-1,3-thiazolidin-4-ones acting as H1-histamine antagonists. Il Farmaco 1999; 54: 579-83.
Ergenç N, Capan G. Synthesis and anticonvulsant activity of new 4-thiazolidone and 4-thiazoline derivatives. Farmaco 1994; 49: 449-51.
Fattovich G, Brollo L, Alberti A, Pontisso P, Giustina G, Realdi G. Long-term follow-up of anti-HBe-positive chronic active hepatitis B. Hepatology 1988; 8: 1651-54.
Ha YM, Park YJ, Kim JA, Park D, Park JY, Lee HJ, Lee JY, Moon HR, Chung HY. Design and synthesis of 5-(substituted benzylidene)thiazolidine-2,4-dione derivatives as novel tyrosinase inhibitors. Eur J Med Chem. 2012; 49: 245-52.
Havrylyuk D, Zimenkovsky B, Lesyk R. Synthesis and anticancer activity of novel nonfused bicyclic thiazolidinone derivatives. Phosphorus Sulfur Silicon Relat Elem. 2009; 184: 638-50.
Korba BE, Gerin JL. Use of a standardized cell culture assay to assess activities of nucleoside analogs against hepatitis B virus replication. Antiviral Res. 1992; 19: 55-70.
Lo C-P, Croxall WJ. 5-Alkoxymethylenerhodanines and their reactions with Rhodanines. J Am Chem Soc. 1954; 76: 4166-69.
Locarnini S, Mason WS. Cellular and virological mechanisms of HBV drug resistance. J Hepatol. 2006; 44: 422-31.
Ma L, Chen J, Liang X, Xie C, Deng C, Huang L, Peng A, Wei Y, Chen L Synthesis and evaluation of 5-benzylidenethiazolidine-2,4-dione derivatives for the treatment of non-alcoholic fatty liver disease. Arch Pharm. 2012; 345: 517.
Piscopo E, Diurno MV, Gagliardi R, Mazzoni O, Veneruso G. Studies on heterocyclic compounds: 1,3-Thiazolidin-4-one derivatives. IV. Biological activity of variously substituted 2,3-diaryl-1,3-thiazolidin-4-ones. Boll Soc Ital Biol Sper. 1989; 65: 853.
Previtera T, Vigorita MG, Basile M, Orsini F, Benetollo F, Bombieri G. 3,3′-Di[1,3-thiazolidine-4-one] system. VI. Structural and conformational studies on configurational isomers with antihistaminic activity. Eur J Med Chem. 1994; 29: 317.
Rawal RK, Prabhakar YS, Katti SB, De Clercq E. 2-(Aryl)-3-furan-2-ylmethylthiazolidin-4-ones as selective HIV-RT inhibitors. Bioorg Med Chem. 2005; 13: 6771.
Sato K, Mori M. Current and novel therapies for hepatitis B virus infection: Mini-review. Med Chem. 2010; 10: 20.
Stachulski AV, Pidathala C, Row EC, Sharma R, Berry NG, Iqbal M, Bentley J, Allman SA, Edwards G, Helm A, Hellier J, Korba BE, Semple JE, Rossignol J-F. Thiazolides as novel antiviral agents. 1. Inhibition of hepatitis B virus replication. J Med Chem. 2011; 54: 4119.
Stray SJ, Bourne CR, Punna S, Lewis WG, Finn MG, Zlotnick A. A heteroaryl dihydropyrimidine activates and can misdirect hepatitis B virus capsid assembly. Proc Natl Acad Sci USA. 2005; 102: 8138.
Wong DKH, Cheung AM, Orourke K, Naylor CD, Detsky AS. Effect of alpha-interferon treatment in patients with heap-titis B e antigen-positive chronic hepatitis B. A meta-analysis. Ann Intern Med. 1993; 119: 312.
Wu Y, Karna S, Choi C H, Tong M, Tai H-H, Na DH, Jang CH, Cho H. Synthesis and biological evaluation of novel thiazolidinedione analogues as 15-hydroxyprostaglandin dehydrogenase inhibitors. J Med Chem. 2011; 54: 5260.
Zidar N,TomaÅ¡ić T, Å ink R, Rupnik V, KovaÄ A, Turk S, Patin D, Blanot D, Martel CC, Dessen A, Premru MM, Zega A, Gobec S, MaÅ¡iÄ LP, Kikelj D. Discovery of novel 5-benzylidenerhodanine and 5-benzylidenethiazolidine-2,4-dione inhibitors of MurD ligase. J Med Chem. 2010; 53: 6584.

