In vitro anti-Candida activity and single crystal X-ray structure of ({(1E)-[3-(1H-imidazol-1-yl)-1-phenylpropylidene]amino}oxy)(4-nitrophenyl)methanone
Abstract
Synthesis, characterization, and anti-Candida activity of ({(E)-[3-(1H-imidazol-1-yl)-1-phenylpropylidene]amino}oxy)(4-nitrophenyl)methanone (4) are reported. Compound 4 showed anti-Candida albicans activity (MIC = 0.6862 µmol/mL) being nearly 5-fold more potent than the gold standard antifungal drug, fluconazole (MIC ƒ 3.265 µmol/mL), on the clinical isolates of Candida albicans. Single crystal X-ray structure of the title compound 4 confirmed its assigned (E)-configuration. The compound crystallizes in the triclinic, P-1 (no. 2), a = 6.4633 (1) Angstrom, b = 11.1063 (2) Angstrom, c = 12.9872 (2) Angstrom, beta = 67.650 (1), beta = 86.415 (1), gamma = 86.776 (1), V = 860.01 (3) Angstrom, Z = 2, R(F) = 0.046, wR(F2) = 0.139, T = 296 K. The crystal structure is stabilized by weak intermolecular C-H...O hydrogen interactions.
Introduction
In recent years, the incidence of serious fungal infections has significantly increased. Such infections are mainly affecting immunocomprimized patients associated with AIDS, organ transplantation, cancer chemotherapy and those using invasive devices, such as such as urinary catheters (Hossain and Ghannoum, 2000). Candida albicans is the most common pathogen in invasive candidiasis, however non-albicans Candida species have become more prevalent as agents of infection (Groll and Walsh, 2001; Singh, 2001; Slavin, 2002).
Additionally, the clinical use of current antifungal drugs can be limited by toxicity, low efficacy rates, and drug resistance (Hossain and Ghannoum, 2000). In order to overcome these serious problems, the development of new antifungal agents has gained great importance (Ghannoum and Rice, 1999; Kathiravan et al., 2012; Moellering, 2011; Pfaller and Diekema, 2007; Sun et al., 2007).
Among the clinically used antifungal agents, azoles were used widely in the treatment of fungal infections. Azole antifungals inhibit P450 14-alpha-demethylase (CYP51) which is an essential enzyme in the sterol biosynthetic pathway in eukaryotes, leading to depletion of ergosterol in fungi (Lamb et al., 2000; Sun et al., 2007). The epidemiological trends have emphasized the increasing importance of the infections caused by resistant fungal species to azoles (Canuto and Rodero, 2002).
In the literature it was reported that some azole antifungals are derived from oxime-containing starting materials (Rossello et al., 2002). Furthermore, many imidazole-containing antifungal agents have a spacer of two carbon atoms between the imidazole pharmacophore and an aromatic moiety, but only limited information about imidazole-containing antifungals having a three-carbon chain between the pharmaco phore and the aromatic moiety is available (Aboul-Enein et al., 2001; Roman et al., 2013).
According to these premises, in this study we aimed to prepare a new imidazole-containing oxime ester, namely ({(E)-[3-(1H-imidazol-1-yl)-1-phenylpropylidene]amino}oxy)(4-nitrophenyl)methanone (4) as potential anti-Candida agent. The target oxime ester 4 has a three-carbon atom linker between the imidazole pharmacophore and the aromatic moiety to be tested as a new imidazole-containing anti-Candida agent. Additionally, the configuration of the title compound 4 was also addressed via exploring its single crystal X-ray structure.
Materials and Methods
General: Melting points were determined on a Gallen-kamp melting point apparatus, and are uncorrected. Infrared (IR) spectra were recorded as KBr disks using the Perkin Elmer FT-IR Spectrum BX apparatus. NMR Spectra were measured in DMSO-d6 on a Bruker NMR spectrometer operating at 500 MHz for 1H and 125.76 MHz for 13C at the Research Center, College of Pharmacy, King Saud University, Saudi Arabia. Chemical shifts are expressed in δ-values (ppm) relative to TMS as an internal standard. Mass spectra were measured on Agilent Triple Quadrupole 6410 QQQ LC/MS with ESI (Electrospray ionization) source. Silica gel TLC (thin layer chromatography) cards from Merck (silica gel precoated aluminium cards with fluorescent indicator at 254 nm) were used for thin layer chromatography. Visualization was performed by illumination with UV light source (254 nm). Fluconazole was obtained from Shouguang-Fukang Pharmaceutical Ltd., Shandong, China. The antifungal discs containing 25 µg fluconazole were purchased from ROSCO (Neo-Sensitabs, Denmark). RPMI 1640 medium was purchased from Sigma-Aldrich Co., USA. Sabouraud Dextrose Agar (SDA) from Difco Laboratories, USA. Potato Dextrose Agar (PDA) was purchased from Eiken Chemical Co. Ltd., Japan. The X-ray diffraction measurements of compound 4 were performed using Bruker SMART APEXII CCD diffractometer. Crystallographic data of compound 4 have been deposited with the Cambridge Crystallographic Data Center (supplementary publication number CCDC-945902). Copies of the data may be obtained free of charge from the Director, CCDC, UK (deposit@ccdc.cam.ac.uk).
Preparation of 3-(1H-imidazol-1-yl)-1-phenylpropan-1-one (2): Acetophenone (2.4 g, 20 mmol), dimethylamine hydrochloride (2.2 g, 27 mmol) and paraformaldehyde (0.81 g, 9 mmol) were refluxed in absolute ethanol (5 mL) in the presence of catalytical amount of concentrated hydrochloric acid (0.1 mL). Reflux of the reaction mixture was continued under stirring for two hours, cooled and acetone (20 mL) was added. The formed Mannich base hydrochloride 1 (3.7 g, 17.4 mmol) was precipitated, filtered, dried and was subsequently dissolved in water (10 mL) and imidazole (2.4 g, 34.8 mmol) was added. The reaction mixture was refluxed for five hours, cooled and the precipitated solid was filtered off to give the ketone 2 (2.7 g, 77%) m.p. 95-97°C (Aboul-Enein et al., 2001) which was pure enough to be used in the next step.
Preparation of (1E)-N-hydroxy-3-(1H-imidazol-1-yl)-1-phenylpropan-1-imine (3): A mixture of the ketone 2 (2.00 g, 10 mmol), hydroxylamine hydrochloride (1.39 g, 20 mmol), and KOH (1.12 g, 20 mmol) in ethanol (10 mL) was refluxed under stirring for 18 hrs. The reaction mixture was cooled to room temperature and the insolubles were filtered off. The filtrate was concentrated under reduced pressure and the residue was poured onto ice-cold water (15 mL). The precipitated solid was filtered, dried, and recrystallized from ethanol to give 1.51 g (70%) of the oxime 3 m.p. 155-157°C as colourless crystals (Attia et al., 2013).
Preparation of ({(E)-[3-(1H-imidazol-1-yl)-1-phenylpropylidene]amino}oxy)(4-nitrophenyl)methanone (4): Ethyl chloroformate (0.67 mL, 0.97 g, 9.00 mmol) was added dropwise to an ice cold stirred solution of triethylamine (1.45 mL, 1.62 g, 16.00 mmol) and 4-nitrobenzoic acid (1.34 g, 8.00 mmol) in dichloromethane (20 mL). The reaction mixture was stirred at room temperature for half an hour, then oxime 3 (1.72 g, 8.00 mmol) was added to the reaction mixture and stirring was continued at room temperature for 18 hours. The organic phase was washed with water (2 x 15 mL), sodium bicarbonate solution (2 x 10 mL) and water (2 x 10 mL). The organic phase was separated, dried (Na2SO4) and evaporated under vacuum to give pale yellow powder of 4. The crude 4 was recrystallized from dimethyl sulfoxide to yield 1.02 g (35%) of the title compound 4 as pale yellow crystals m.p. 130-132°C. IR (KBr): d (cm-1) 3115, 2713, 1743 (C=O), 1645 (C=N), 1520, 1257, 749; 1H NMR (DMSO-d6): d (ppm) = 3.55 (t, J = 6.7 Hz, 2H, -CH2-CH2-N), 4.32 (t, J = 6.7 Hz, 2H, -CH2-CH2-N), 6.78 (s, 1H, -N-CH=CH-N=), 7.17 (s, 1H, -N-CH=CH-N=), 7.50-7.59 (m, 4H, -N-CH=N-, Ar-H), 7.78 (d, J = 7.3 Hz, 2H, Ar-H.), 8.27 (d, J = 8.8 Hz, 2H, Ar-H), 8.40 (d, J = 8.8 Hz, 2H, Ar-H); 13C NMR (DMSO-d6): d 30.1 (-CH2-CH2-N), 43.0 (-CH2-CH2-N), 119.4 (-N-CH=CH-N=), 123.9, 127.3, 128.5, 128.8, 130.9, 131.2, 132.8 (-N-CH=CH-N=, Ar-CH, Ar-C), 133.8, 137.2 (-N-CH=N-, Ar-C), 150.4 (Ar-C.), 161.4 (C=N), 165.1 (C=O); MS m/z (ESI): 365.1 [M + 1]+.
Crystal structure determination: Slow evaporation of the pure ester 4 in dimethyl sulfoxide yielded its pale yellow single crystals. A yellow block-shaped single crystal of suitable size, 0.40 mm X 0.54 mm X 0.78 mm, was selected for X-ray diffraction analysis. Data were collected on a Bruker APEX-II CCD area diffractometer equipped with graphite monochromatic CuK\α radia tion (λ=1.54178 beta) at 296 K. Cell refinement and data reduction were done by Bruker SAINT (Brucker, 2009); program used to solve structure and refine structure is SHELXTL (Sheldrick, 2008). The final refinement was performed by full-matrix least-squares techniques with anisotropic thermal data for non hydrogen atoms on F2. All the hydrogen atoms were placed in calculated positions and constrained to ride on their parent atoms. Multi-scan absorption correction was applied by use of SADABS software (Brucker, 2009). The crystallographic data and refinement information are summarized in Table I.
Table I: Crystallographic data and refinement information
| Empirical formula | C19H16N4O4 |
|---|---|
| Formula weight | 364.36 |
| Temperature (K) | 296 |
| Crystal system | Triclinic |
| Space group | P-1 |
| Cu K alpha radiation, λ | 1.54178 Angstrom |
| a (Angstrom) | 6.4633 (1) |
| b (Angstrom) | 11.1063 (2) |
| c (Angstrom) | 12.9872 (2) |
| alpha (º) | 67.650 (1) |
| beta (º) | 86.415 (1) |
| gamma (º) | 86.776 (1) |
| V (Angstrom3) | 860.01 (3) |
| Z | 2 |
| F(000) | 380 |
| Theta range for data collection (º) | 4.5-69.9 |
| Â (mm-1) | 0.84 |
| Density (calc.) (g/cm3) | 1.407 |
| Crystal shape and color | Block, yellow |
| Crystal size (mm3) | 0.78 x 0.54 x 0.40 |
| h / k / l | -7,7/-9,12/ -15,15 |
| Measured reflections | 10585 |
| Independent reflections | 3129 [Rint = 0.025] |
| Reflections with I > 2alpha(I) | 2815 |
| Goodness-of-fit on F2 | 1.05 |
| R[F2 > 2alpha(F2)] | 0.046 |
| wR(F2) | 0.139 |
|
Δρmax (e Agstrom3)
|
0.29 |
| Δρmax (e Agstrom3) | -0.26 |
Anti-Candida agents: Stock solution (1,000 µg/mL) of test compound 4 as well as fluconazole were prepared in 100% dimethylsulfoxide and were diluted with sterile distilled water. Fluconazole antifungal discs were stored at -80°C until used.
Organisms: Two clinical isolates of Candida species were kindly provided by King Khaled Hospital, Riyadh, Saudi Arabia, one identified as C. albicans and the other as C. tropicalis. The isolates were preserved in glycerol broth at -80°C until used.
Media: PDA and SDA were used for routine subculturing of the Candida strains while RPMI 1640 medium supplemented with L-glutamine was buffered to pH 7.0 with 0.165 M 3-(N-morpholino)propane sulfonic acid (MOPS) was used for broth microdilution assay.
Broth microdilution testing: Preparation of inocula was performed in accordance with CLSI documents M27-A2 (CLSI, 2002) with RPMI 1640 medium. Isolates of Candida species were subcultured at 35°C for 48 hours on PDA plates. Yeast cells were recovered and suspended in 5 mL of sterile saline. The turbidity of each Candida cell suspension was adjusted to a 0.5 McFarland standard (1.3 x 106 to 5.3 x 106 CFU/mL) at a wavelength of 530 nm according to the reported method (CLSI, 2002). Each suspension was diluted with RPMI 1640 medium (1:1000) to give a final inoculum of 1.3 x 103 to 5.3 x 103 CFU/mL.
Disk diffusion assay: As reported previously (CLSI, 2005), cell suspensions of the Candida isolates under test were adjusted to 5 x 106 CFU/mL (0.5 McFarland standard). A 100 µL suspension of each tested strain was uniformly plated onto SDA plates. Whatman filter paper disks with a diameter of 6 mm were impregnated with 1,000 µg of the test compound 4. The disks were allowed to dry then they were placed onto the surface of the inoculated agar plates together with fluconazole antifungal discs. The plates were then incubated at 35°C and diameters of inhibition zones were measured at 24 hours.
Antifungal susceptibility studies: 100 µL Aliquots of the prepared Candida inocula were added to each well of the 96-well microdilution plates; each well contained 100 µL of twofold serial dilutions of fluconazole or test compound 4 (1 µg/mL to 500 µg/mL) in RPMI 1640 medium. The plates was incubated at 35 °C for 48 hours then the turbidity of each well was measured at 490 nm with a microplate ELISA reader. The MICs of the Candida species were recorded as the lowest concentration at which a prominent decrease (50%-80%) in turbidity relative to the turbidity of the growth control.
Result and Discussion
Scheme 1 illustrates the synthetic strategy which was successfully adopted to achieve the target compound 4. Thus, acetophenone was converted to the pivotal ketone 2 via Mannich reaction and subsequent alkylation of imidazole by the formed Mannich base hydrochloride 1 to give the ketone 2. Compound 2 was allowed to react with hydroxylamine hydrochloride in the presence of potassium hydroxide to yield the penultimate oxime 3. Subsequently, the hydroxyl group of compound 3 was esterified with 4-nitrobenzoic acid in the presence of ethyl chloroformate and triethylamine to give the title compound 4. The structure of compound 4 was confirmed via IR, 1H NMR, 13C NMR and mass spectral data. Reagents and conditions: i) HN(CH3)2.HCl, (CH2O)n, concentrated HCl, ethanol, reflux, 2 hours; ii) Imidazole, water, reflux, 5 hours; iii) H2NOH.HCl, KOH, ethanol, reflux, 18 hours; iv) Ethyl chloroformate, triethylamine, 4-nitrobenzoic acid, DCM, rt, 18 hours.
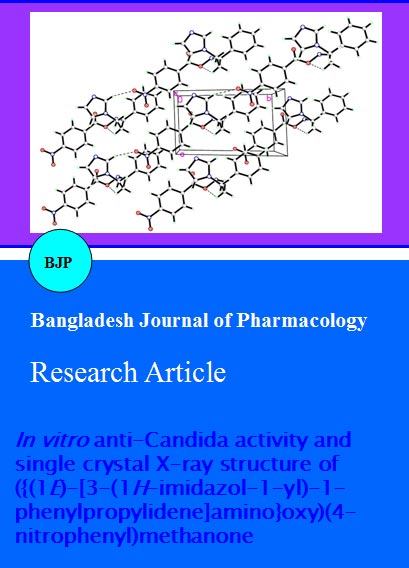
Single crystal X-ray crystallography is undoubtlessly a decisive analytical tool which can confirm the configuration of the imine double bond in the target compound 4. Fortunately, we have succeeded to get single crystals of compound 4 which were suitable for X-ray crystallography. Accordingly, the assigned (E)-configuration of compound 4 was confirmed via its single crystal X-ray structure (Figure 1).
Scheme 01: Synthesis of the target compound 4
Figure 1: ORTEP diagram of the title compound 4 drawn at 50% ellipsoids for non-hydrogen atoms
The (E)-isomer of the title compound 4, as a kinetically favored isomer, was formed over the (Z)- isomer due to steric factors. The torsion angle between C8-N2-O4-C7 is -154.14 (12). The single bond N2-O4 is clearly characterized by the distance of 1.4422(16) Ã…. The double bond of C8=N2 is characterized by the distance of 1.2784(19) Angstrom. The molecules packing in the crystal structure is stabilized by one weak intermolecular interaction (Figure 2, Table II). There is also one intramolecular H-bond in the molecule (Figure 1, Table II). The selected bond lengths, bond angles and bond torsion angles are listed in Table III.
Figure 2: Crystal packing showing intermolecular C-H•••O hydrogen bonds as dashed lines
Table II: Hydrogen-bond geometry (Angstrom)
| D-H...A | D-H | H...A | D...A | D-H...A |
|---|---|---|---|---|
| C15-H15A..O4 | 0.9700 | 2.2400 | 2.663 (2) | 105.00 |
| C17-H17A..O1i | 0.9300 | 2.5200 | 3.341 (3) | 148.00 |
| Symmetry code: (i) x+1, y−1, z. | ||||
Table III: Selected geometric parameters (Angstrom, °)
| O1-N1 | 1.219 (2) | N2-C8 | 1.2785 (19) |
|---|---|---|---|
| O2-N1 | 1.2124 (19) | N3-C16 | 1.453 (2) |
| O3-C7 | 1.1902 (18) | N3-C17 | 1.355 (2) |
| O4-N2 | 1.4422 (16) | N3-C19 | 1.371 (2) |
| O4-C7 | 1.3473 (19) | N4-C17 | 1.309 (3) |
| N1-C3 | 1.469 (2) | N4-C18 | 1.414 (4) |
| N2-O4-C7 | 112.12 (10) | N1-C3-C4 | 118.10 (14) |
| O1-N1-O2 | 123.17 (17) | O3-C7-O4 | 125.13 (14) |
| O1-N1-C3 | 117.86 (14) | O3-C7-C6 | 124.25 (15) |
| O2-N1-C3 | 118.98 (14) | O4-C7-C6 | 110.60 (11) |
| O4-N2-C8 | 110.00 (11) | N2-C8-C9 | 113.74 (12) |
| C16-N3-C17 | 126.51 (15) | N2-C8-C15 | 126.14 (13) |
| C16-N3-C19 | 127.82 (16) | N3-C16-C15 | 112.42 (13) |
| C17-N3-C19 | 105.49 (16) | N3-C17-N4 | 112.12 (19) |
| C17-N4-C18 | 104.9 (2) | N4-C18-C19 | 108.6 (2) |
| N1-C3-C2 | 119.16 (12) | N3-C19-C18 | 108.9 (2) |
Fluconazole was used in clinics as a first line of treatment for fungal infections especially those caused by C. albicans, but its extensive medical use led to the emergence of resistance (Pfaller et al., 2010). The in vitro anti-Candida activity of compound 4 was evaluated against two clinical isolates of Candida, namely C. albicans and C. tropicalis. The clinical isolates of C. albicans were considered practically insensitive to fluconazole (MIC ˃3.265 µmol/mL). The obtained data, expressed as diameter of the inhibition zone (DIZ) and minimum inhibition concentration (MIC) for the test compound 4 and for the reference standard, fluconazole, are shown in Table IV.
Table IV: Anti-candida activity of compound 4 and fluconazole against C. albicans and C. tropicalis
| C. albicans | C. albicans | C. tropicalis | C. tropicalis | |
|---|---|---|---|---|
| DIZ ± SDa | MIC (µmol/mL)b | DIZ ± SDa | MIC (µmol/mL)b | |
| Compound 4 | 13 ±1.2 | 0.6862 | 17 ± 1.0 | 0.3431 |
| Fluconazole | 15 ± 0.5 | >3.2651 | 16 ± 0.5 | 0.2024 |
| aThe arithmetic mean of the inhibition zone diameters in mean ± standard deviation in mm; bThe lowest concentration of the compound that produced 50-80% microbial growth inhibition (umol/mL) | ||||
Compound 4 exhibited a good anti-Candida activity with DIZ = 13 and 17 mm against C. albicans and C. tropicalis, respectively. Consequently, the minimum inhibition concentration assay was performed in order to evaluate the potency of compound 4 as a new anti-Candida agent. Compound 4 was about five times more potent than fluconazole, towards C. albicans while it was nearly equipotent with fluconazole towards C. tropicalis on the clinical isolates of Candida.
Conclusion
The synthesized new compound, ({(E)-[3-(1H-imidazol-1-yl)-1-phenylpropylidene]amino}oxy)(4-nitrophenyl)methanone (4) is nearly five-fold more potent than fluconazole, towards clinical isolates of Candida albicans.
Acknowledgement
The authors would like to extend their sincere appreciation to the Dean of Scientific Research at King Saud University.